what is the electron configuration for iron|electron configuration guide : iloilo Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .
Dora the Explorer is a Nick Jr. show created by Chris Gifford, Valerie Walsh Valdes, and Eric Weiner. This play-along, animated adventure series stars Dora, a seven-year-old Latina heroine who asks preschoolers for their help on her adventures. Along the way, they'll meet friends, overcome obstacles and learn a little Spanish! — NickJr.com The .Understanding the SIM card slot on a Dell Latitude laptop. The SIM card slot on a Dell Latitude laptop is a small space designed to hold a SIM card, which is a small chip used for cellular connectivity. This feature allows you to access cellular data on your laptop, even when Wi-Fi is not available.
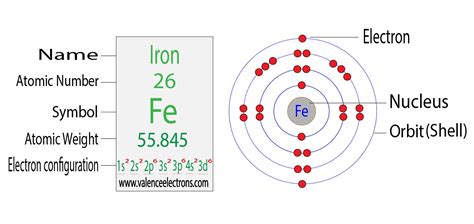
what is the electron configuration for iron,In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
In order to write the Calcium electron configuration we first need to know the .Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .
In order to write the Mg electron configuration we first need to know the .
Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .
Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .In order to write the Argon electron configuration we first need to know the .How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element .
Mar 23, 2023 Learn about the electronic configuration of iron, a transition metal with an atomic number 26 and a symbol Fe. Find out how iron has two .
Electronic Configuration of Iron. Iron, a transition element in Periodic Group VIII, is the fourth most abundant element in the Earth’s crust in terms of mass, and the second .
Indeed, 2 + 2 + 6 + 2 +6 +6 +2 = 26, the atomic number of Fe. Answer link. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. What we have is: Its core . In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons). When we write the configuration, we'll .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic .
1 Answer. mrpauller.weebly.com. Jun 28, 2016. 1s22s22p63s23p64s23d6. Explanation: To write an electron configuration for any element you'll need to apply the .
To write the orbital diagram for the Iron (Fe) first we need to write the electron configuration for just Fe. To do that we need to find the number of elect. The ground state electron configuration of Fe is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^6"4s"^2" For all but about 20 transition metals, the Aufbau diagram is a useful tool that helps to determine the .
That is, you could write Na: 1s 2 2s 2 2p 6 3s 1 or [Ne]3s 1 noting that [Ne]=1s 2 2s 2 2p 6. Figure 7.2.3 7.2. 3: For sodium it is better to use the convention of expressing the core electrons with that of a Nobel gas. Figure 7.2.4 7.2. .
what is the electron configuration for iron electron configuration guide The electron configuration for the iron (III) ion is: 1s2s22p63s23p63d5. The element iron, Fe, has the atomic number 26, which is the number of protons in its atomic nuclei. A neutral iron atom has 26 protons and 26 electrons. In order to form a 3+ ion, it must lose three electrons.what is the electron configuration for ironFor example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence .Orbital diagram. Iron electron configuration. ← Electronic configurations of elements. Fe (Iron) is an element with position number 26 in the periodic table. Located in the IV period. Melting point: 1535 ℃. Density: 7.87 g/cm 3 . Electronic configuration of the Iron atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 .

Iron has 26 electrons so its normal electron configuration would be: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. When we make a 3+ ion for Iron, we need to take the electrons from the outermost shell first so that would be the 4s shell NOT the 3d shell: Fe 3+ 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. One other note on writing electron configurations: A short cut.
This leaves Iron ( Fe) with 8 valance electrons. Two valance electrons are in the 4 s orbital. These are the first electrons that Iron tends to lose. giving a charge of +2. A half filled 3d orbital is somewhat stable so the 6 3d electrons are not stable. losing one 3d electron leaves 5 3d electrons which is half filled electron sub shell, which .
For this, iron ion (Fe 2+) has a total of fourteen valence electrons. Again, the iron atom donates two electrons in the 4s orbital and an electron in the 3d orbital to convert an iron ion (Fe 3+ ). Fe – 3e – → Fe 3+. Hare, the electron configuration of iron ion (Fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. The number of electrons in the valence shell of iron (Fe) can be used to calculate the electronic configuration of the various oxidation states. Fe2+ F e 2 +: Iron now has an electron configuration of [Ar]3d6 [ A r] 3 d 6 after losing two electrons from its 4s orbital. The iron ion acquires a 2+ charge as a result.
Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Its 26 electrons are arranged in the configuration [Ar]3d 6 4s 2, of which the 3d and 4s electrons are relatively close in energy, and thus .

1 Answer. Christopher P. electron configuration guide 1 Answer. Christopher P. In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .
For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdf notation) is written as 1s 1 and read as “one-s-one. . The element is iron, Fe. b) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 7. The element is Rhodium, Rh. c) 1s 2 2s 2 2p 6 3s 2 3p 4.
Of the elements discussed in the video, iron is most like Sc, but iron has 5 more electrons in the last subshell which is filled with electrons making its configuration end in 3d6 rather than 3d1. Hope this helps! Answer link. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 To write an electron configuration for any element you'll need to apply the Aufbau .
The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6.This structure is called an electron configuration and is unique to hydrogen. Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells contain one s orbital which can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). The electronic configuration of the Iron in its ground state is 1s2 2s2 2p6 3s2 3p6 3d6 4s2 and is also written as [Ar] 3d6 4s2. When an Iron atom donates two of its electrons, it attains a charge of +2. Any element that donates electrons gets a positive charge, whereas the elements that accept electrons gain a negative charge. .
what is the electron configuration for iron|electron configuration guide
PH0 · iron 2+ electron configuration
PH1 · how many electrons does iron have
PH2 · full electron configuration cu
PH3 · electron configuration guide
PH4 · electron configuration for fe+3
PH5 · electron configuration chart
PH6 · condensed electron configuration fe
PH7 · abbreviated electron configuration for iron
PH8 · Iba pa